Volume 19, Issue 2 (Mar-Apr 2025)
mljgoums 2025, 19(2): 34-37 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hasanein P, Javadi Hedaiat Abad F, Bohlooli M, Khajeh M, Esmaielzadeh Bahabadi S, Poormolaei N. Attenuation of glucose mediated DNA glycation by Tamarix aphylla extract. mljgoums 2025; 19 (2) :34-37
URL: http://mlj.goums.ac.ir/article-1-1862-en.html
URL: http://mlj.goums.ac.ir/article-1-1862-en.html
Parisa Hasanein1 

 , Fahime Javadi Hedaiat Abad2
, Fahime Javadi Hedaiat Abad2 

 , Mousa Bohlooli3
, Mousa Bohlooli3 

 , Mostafa Khajeh4
, Mostafa Khajeh4 

 , Sedigheh Esmaielzadeh Bahabadi2
, Sedigheh Esmaielzadeh Bahabadi2 

 , Neda Poormolaei2
, Neda Poormolaei2 




 , Fahime Javadi Hedaiat Abad2
, Fahime Javadi Hedaiat Abad2 

 , Mousa Bohlooli3
, Mousa Bohlooli3 

 , Mostafa Khajeh4
, Mostafa Khajeh4 

 , Sedigheh Esmaielzadeh Bahabadi2
, Sedigheh Esmaielzadeh Bahabadi2 

 , Neda Poormolaei2
, Neda Poormolaei2 


1- Department of Biology, School of Basic Sciences, University of Zabol, Zabol, Iran , parisa.hasanein@gmail.com
2- Department of Biology, School of Basic Sciences, University of Zabol, Zabol, Iran
3- Department of Biology, School of Basic Sciences, University of Zabol, Zabol, Iran; Department of Cell and Molecular Sciences, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
4- Department of Chemistry, School of Basic Sciences, University of Zabol, Zabol, Iran
2- Department of Biology, School of Basic Sciences, University of Zabol, Zabol, Iran
3- Department of Biology, School of Basic Sciences, University of Zabol, Zabol, Iran; Department of Cell and Molecular Sciences, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
4- Department of Chemistry, School of Basic Sciences, University of Zabol, Zabol, Iran
Full-Text [PDF 463 kb]
(292 Downloads)
| Abstract (HTML) (1190 Views)
Figure 2 illustrates the CD spectra obtained for all experimental conditions. The control-DNA sample exhibited a negative ellipticity peak of -8 millidegrees (mdeg) at a wavelength of 255 nm and a positive ellipticity peak of +17 mdeg at 275 nm. The samples containing DNA + T. aphylla, DNA + Glc + T. aphylla, and DNA + Glc displayed negative ellipticity peaks of -6.3, -4.4, and -3.2 mdeg, respectively, all shifted to a wavelength of 245 nm. These samples also presented positive ellipticity peaks at 275 nm with magnitudes of 16.9, 12.1, and 11.9 mdeg, respectively.
The fluorescence emission spectra obtained for all experimental conditions are presented in Figure 3. As illustrated therein, the highest fluorescence intensity was observed in the DNA + Glc group. These findings indicate that the presence of T. aphylla resulted in a reduction of the fluorescence emission of DNA when compared to the DNA + Glc group.
Electrophoretic analyses of all experimental groups are illustrated in Figure 4. The DNA + Glc group exhibited the highest electrophoretic mobility in comparison to other groups. Notably, the co-incubation of T. aphylla extract with DNA and Glc resulted in a substantial reduction in electrophoretic mobility, as demonstrated by the electrophoresis results presented in Figure 4.
Discussion
Despite ongoing efforts to elucidate the structural and functional alterations in proteins resulting from glycation, the non-enzymatic glycation of eukaryotic DNA has been the subject of comparatively limited in-depth investigation. Existing literature has documented the contribution of accumulating AGEs on both proteins and DNA to the development of diabetes mellitus and age-related pathologies (1,13). The DNA glycation process ultimately culminates in structural alterations, strand breaks, and the induction of mutations (20). Several compounds have demonstrated inhibitory effects on the glycation process, including vitamin B6 (21), aminoguanidine (22), quercetin (23), and aspirin (24). Research into agents that inhibit glycation is of significant importance for identifying their potential benefits in preventing not only the complications associated with diabetes mellitus but also certain age-related neurodegenerative disorders.
In recent scientific inquiry, there has been a significant focus on herbal medicines possessing both antiglycation and antioxidant properties as potential agents for preventing and mitigating the complications associated with the accumulation of AGEs (4). Illustratively, the seed extract of Nigella sativa has been demonstrated to inhibit protein glycation in bovine serum albumin and has also exhibited a substantial capacity for protecting against DNA damage (25). Based on the UV-Vis spectrophotometry results obtained in the present investigation, the inclusion of T. aphylla extract led to a reduction in the absorbance of DNA incubated with Glc. Prior research indicates that the UV-Vis absorbance of glycated DNA is augmented due to the partial unwinding of the double helical structure and the consequent exposure of chromophoric bases (26). Furthermore, existing literature has documented that Glc induces alterations in the biophysical and chemical characteristics of DNA (13,14). For instance, DNA exposed to Glc demonstrates hyperchromicity, a reduction in melting temperature (Tm), and an increase in emission intensity, exhibiting a temporal dependence (20). The present investigation constitutes an in vitro study that reports, for the first time, the impact of a T. aphylla extract on the structural alterations of glycated DNA. Therefore, considering the aforementioned direct effects of Glc on DNA structure, it is plausible that T. aphylla diminishes the UV-Vis absorbance of DNA through a dual mechanism involving direct interactions with the DNA molecule and indirect effects mediated by its established antioxidant and reactive oxygen species (ROS) scavenging activities. Given that ROS serve as potent mediators of cellular stress originating from the auto-oxidation of sugars (27), the antioxidant capacity of T. aphylla may contribute to the observed effects.
Based on the findings obtained from fluorescence spectroscopy, the fluorescence emission of the DNA + Glc + T. aphylla sample exhibited a reduction in intensity when compared to the DNA + Glc sample. Prior research indicates that glycated DNA has an excitation wavelength of 400 nm and an emission wavelength of 290 nm (28). Consequently, the observed decrease in fluorescence intensity suggests that the presence of T. aphylla exerts an inhibitory effect on DNA glycation and associated structural alterations. These results are in agreement with a previous investigation from our laboratory, which demonstrated the inhibitory effect of 3-β-hydroxybutyrate on the fluorescence intensity of DNA + Glc (13).
The results obtained from CD spectroscopy indicated that the negative and positive ellipticity bands in the CD spectra of the DNA + Glc exhibited an increase and a decrease in magnitude, respectively, when compared to the CD spectra of control-DNA. This observation is consistent with the findings reported in prior published research (26). Moreover, the DNA sample demonstrated fewer structural alterations when incubated in the presence of both Glc and T. aphylla extract. Consequently, the co-incubation of this plant extract with DNA and Glc appears to mitigate structural alterations and, ultimately, the formation of DNA-AGEs. These spectroscopic findings are in agreement with the results obtained from UV-Vis spectrophotometry.
Electrophoretic analysis revealed that DNA + Glc exhibited increased mobility compared to the control-DNA, a finding consistent with prior studies (13,25). Conversely, the electrophoretic mobility of DNA samples incubated with both Glc and T. aphylla was lower in this investigation. These results suggest that the presence of T. aphylla exerts an inhibitory effect on the extent of structural alterations and damage to DNA compared to DNA + Glc.
Conclusion
While ongoing research endeavors are dedicated to elucidating the structural and functional alterations in proteins resulting from glycation, the non-enzymatic glycation of eukaryotic DNA has been the subject of comparatively limited in-depth investigation. The present study offers compelling and evidence-based findings regarding the advantageous effects of T. aphylla on DNA glycation and associated structural modifications in the presence of Glc. Specifically, T. aphylla demonstrated an inhibitory influence on Glc-induced DNA structural alterations and the subsequent formation of AGEs. A reduction in the formation of AGEs was demonstrated through UV-Vis and fluorescence spectrometry. The incubation of the plant extract with DNA and Glc resulted in a decrease in UV-Vis absorbance and fluorescence intensity by approximately 33.6% and 26.6%, respectively. These observed effects appear to be mediated through both direct interactions with DNA and indirect mechanisms stemming from the established antioxidant and ROS scavenging activities of T. aphylla. Moreover, the observed effects may be attributed to the extract's potential to preserve the global conformation of the DNA molecule. This implies a possible interaction, such as stacking, with glycated nucleotides, which may direct ligands towards the glycated core. This interaction could consequently counteract the impact of inter-strand cross-links formed within the double-stranded DNA. Regardless of the precise underlying mechanisms, the findings of the current investigation warrant further in-depth studies to elucidate the detailed molecular mechanisms involved.
Acknowledgement
The authors would like to thank the staff of the central laboratory for their invaluable support in the execution of this research project.
Funding sources
This research was financially supported by a grant (Grant number: IR-UOZ-GR 9452) from the University of Zabol, Zabol, Iran.
Ethical statement
Given that this research was conducted in vitro and involved the analysis of plant DNA utilizing various biophysical methodologies, it was exempt from requiring an ethical statement.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
MB: Study design; FJHA and NP: Executing the experimental procedures; PH and MKh: Data analysis; PH and NP: Writing initial draft of the manuscript; SEB: Conducting the botanical authentication of the plant material. All listed authors have critically reviewed and approved the final version of this manuscript prior to its submission.
Data availability statement
Data can be provided upon request.
Full-Text: (10 Views)
Introduction
Chronic hyperglycemia initiates non-enzymatic DNA glycation, a sequential series of chemical reactions involving the interaction between amino groups of nucleic acids and carbonyl groups of reducing sugars (1). The end products of this process are “advanced glycation end products” (AGEs), which are recognized as significant contributors to the development of diabetic complications and have been shown to be elevated in both urine and tissues in animal models of the disease (2), coupled with the pathogenesis of other conditions, including Parkinson's disease, Alzheimer's disease, and the aging process (3).
Herbal medicinal products exhibiting both antiglycation and antioxidant activities are considered to be of critical importance in the prevention and mitigation of AGE-mediated complications associated with diabetes mellitus (4). Tamarix aphylla (T. aphylla), a medium-sized arboreal species widely distributed across the Middle East and regions of Southern and Western Asia (4), possesses a substantial history of application in traditional medical practices. Within traditional medicine, T. aphylla has been recommended for the management of a diverse range of ailments and disorders, including its use as an antipyretic, antimicrobial, antifungal, analgesic, antirheumatic, and anti-inflammatory agent (5-7).
T. aphylla represents a potentially valuable natural resource due to its high content of polyphenolic compounds, including flavonoids, phenolic acids, tannins, and coumarins, thus positioning it as a prospective source for the development of novel antioxidant pharmaceuticals (7-9). Moreover, the T. aphylla leaf extract has demonstrated hypoglycemic activity under diabetic conditions and may offer protective effects against diabetic complications associated with elevated blood glucose (Glc) levels (10). Consequently, the current investigation was undertaken to ascertain the antiglycation potential of T. aphylla extract in the presence of Glc utilizing UV-Vis spectrophotometry, fluorescence spectroscopy, CD spectroscopy, and agarose gel electrophoresis.
Methods
Chemicals
The following chemical reagents and biological materials were procured from Sigma-Aldrich (USA): β-D Glc, DNA derived from calf thymus, agarose, ethidium bromide, acetoacetate (AA), sodium dihydrogen orthophosphate, disodium hydrogen phosphate, ethylenediaminetetraacetic acid (EDTA), nitro-blue tetrazolium (NBT), sodium chloride, and Tris-hydrochloride (Tris-HCl).
Preparation of Advanced Glycation End Product (AGE)-DNA
DNA (25 μg/mL) and dextrorotatory Glc (D-Glc) (130 mM) were combined in a 200 mM sodium phosphate buffer solution adjusted to a pH of 7.4. This mixture was prepared with and without the addition of T. aphylla extract at a final concentration of 0.05%. Following a four-week incubation period, the resulting mixtures underwent dialysis against the sodium phosphate buffer for 48 hours to eliminate unbound molecular species. Subsequently, the samples were stored at a temperature of -30 °C. The control sample consisted of DNA incubated in the absence of both Glc and the plant extract. The protocol for the preparation of AGE-DNA was conducted in accordance with previously established methodologies and our prior published research (11-13).
Ultraviolet-Visible (UV-Vis) analysis
UV-Vis spectroscopic analyses were performed using a Cary spectrophotometer (UV-2100, Rayleigh, China) following previously published methodologies (13,14). The absorbance spectra of the samples were recorded across a wavelength range of 200 to 600 nm.
Fluorescence analysis
Fluorescence analyses were conducted following previously published protocols (12,13,15) utilizing a spectrofluorophotometer (RF-5301-PC, Japan) at excitation wavelengths of 290 and 400 nm.
Circular Dichroism (CD) analysis
For the conduction of CD spectroscopic analyses, a spectropolarimeter (Jasco J-815, Japan) was utilized across a wavelength range of 220 to 400 nm. The experimental procedure adhered to previously published methodologies (12,13).
Agarose Gel Electrophoresis
DNA samples were subjected to agarose gel electrophoresis for a duration of 2 hours at a constant current of 30 mA, utilizing a 0.8% agarose gel matrix. The running buffer consisted of 40 mM Tris-acetate and 2 mM EDTA. Following staining with ethidium bromide, DNA bands were visualized through UV illumination (13,16).
Plant material and preparation of extract
Fresh leaves of T. aphylla L. were harvested in April 2021 from Zabol, Iran. Botanical identification and authentication of the plant material were performed by Dr. Esmaeilzadeh at the Department of Biology at University of Zabol. The extraction procedure adhered to the methodology outlined in prior research (17). Specifically, the harvested leaves were subjected to shade drying at a temperature range of 30 to 35 °C. Following the drying process, the leaves were mechanically pulverized into a coarse powder using an automated blender. The resultant powdered material (3.24 kg) underwent maceration in a hydroethanolic solvent system (500 mL, 50% v/v ethanol and water) at ambient temperature (26 ± 1 °C) for 48 hours, with intermittent agitation. The obtained filtrate was subsequently concentrated under reduced pressure at a temperature of 40 °C until the extraction solvent was completely removed. This process yielded a dark green, soluble crude residue (Approximately 214.27 g, 6.61% w/w extraction yield).
Results
Ultraviolet-Visible (UV-Vis) spectroscopy
Figure 1 illustrates the UV-Vis spectra obtained for all experimental conditions, encompassing the control-DNA, DNA + T. aphylla, DNA + Glc + T. aphylla, and DNA + Glc. The data presented in this figure indicate that the highest absorbance value was observed for the DNA + Glc. Notably, the inclusion of T. aphylla in the Glc-containing sample resulted in a reduction of approximately 42.85% in absorbance, as depicted in Figure 1.
Chronic hyperglycemia initiates non-enzymatic DNA glycation, a sequential series of chemical reactions involving the interaction between amino groups of nucleic acids and carbonyl groups of reducing sugars (1). The end products of this process are “advanced glycation end products” (AGEs), which are recognized as significant contributors to the development of diabetic complications and have been shown to be elevated in both urine and tissues in animal models of the disease (2), coupled with the pathogenesis of other conditions, including Parkinson's disease, Alzheimer's disease, and the aging process (3).
Herbal medicinal products exhibiting both antiglycation and antioxidant activities are considered to be of critical importance in the prevention and mitigation of AGE-mediated complications associated with diabetes mellitus (4). Tamarix aphylla (T. aphylla), a medium-sized arboreal species widely distributed across the Middle East and regions of Southern and Western Asia (4), possesses a substantial history of application in traditional medical practices. Within traditional medicine, T. aphylla has been recommended for the management of a diverse range of ailments and disorders, including its use as an antipyretic, antimicrobial, antifungal, analgesic, antirheumatic, and anti-inflammatory agent (5-7).
T. aphylla represents a potentially valuable natural resource due to its high content of polyphenolic compounds, including flavonoids, phenolic acids, tannins, and coumarins, thus positioning it as a prospective source for the development of novel antioxidant pharmaceuticals (7-9). Moreover, the T. aphylla leaf extract has demonstrated hypoglycemic activity under diabetic conditions and may offer protective effects against diabetic complications associated with elevated blood glucose (Glc) levels (10). Consequently, the current investigation was undertaken to ascertain the antiglycation potential of T. aphylla extract in the presence of Glc utilizing UV-Vis spectrophotometry, fluorescence spectroscopy, CD spectroscopy, and agarose gel electrophoresis.
Methods
Chemicals
The following chemical reagents and biological materials were procured from Sigma-Aldrich (USA): β-D Glc, DNA derived from calf thymus, agarose, ethidium bromide, acetoacetate (AA), sodium dihydrogen orthophosphate, disodium hydrogen phosphate, ethylenediaminetetraacetic acid (EDTA), nitro-blue tetrazolium (NBT), sodium chloride, and Tris-hydrochloride (Tris-HCl).
Preparation of Advanced Glycation End Product (AGE)-DNA
DNA (25 μg/mL) and dextrorotatory Glc (D-Glc) (130 mM) were combined in a 200 mM sodium phosphate buffer solution adjusted to a pH of 7.4. This mixture was prepared with and without the addition of T. aphylla extract at a final concentration of 0.05%. Following a four-week incubation period, the resulting mixtures underwent dialysis against the sodium phosphate buffer for 48 hours to eliminate unbound molecular species. Subsequently, the samples were stored at a temperature of -30 °C. The control sample consisted of DNA incubated in the absence of both Glc and the plant extract. The protocol for the preparation of AGE-DNA was conducted in accordance with previously established methodologies and our prior published research (11-13).
Ultraviolet-Visible (UV-Vis) analysis
UV-Vis spectroscopic analyses were performed using a Cary spectrophotometer (UV-2100, Rayleigh, China) following previously published methodologies (13,14). The absorbance spectra of the samples were recorded across a wavelength range of 200 to 600 nm.
Fluorescence analysis
Fluorescence analyses were conducted following previously published protocols (12,13,15) utilizing a spectrofluorophotometer (RF-5301-PC, Japan) at excitation wavelengths of 290 and 400 nm.
Circular Dichroism (CD) analysis
For the conduction of CD spectroscopic analyses, a spectropolarimeter (Jasco J-815, Japan) was utilized across a wavelength range of 220 to 400 nm. The experimental procedure adhered to previously published methodologies (12,13).
Agarose Gel Electrophoresis
DNA samples were subjected to agarose gel electrophoresis for a duration of 2 hours at a constant current of 30 mA, utilizing a 0.8% agarose gel matrix. The running buffer consisted of 40 mM Tris-acetate and 2 mM EDTA. Following staining with ethidium bromide, DNA bands were visualized through UV illumination (13,16).
Plant material and preparation of extract
Fresh leaves of T. aphylla L. were harvested in April 2021 from Zabol, Iran. Botanical identification and authentication of the plant material were performed by Dr. Esmaeilzadeh at the Department of Biology at University of Zabol. The extraction procedure adhered to the methodology outlined in prior research (17). Specifically, the harvested leaves were subjected to shade drying at a temperature range of 30 to 35 °C. Following the drying process, the leaves were mechanically pulverized into a coarse powder using an automated blender. The resultant powdered material (3.24 kg) underwent maceration in a hydroethanolic solvent system (500 mL, 50% v/v ethanol and water) at ambient temperature (26 ± 1 °C) for 48 hours, with intermittent agitation. The obtained filtrate was subsequently concentrated under reduced pressure at a temperature of 40 °C until the extraction solvent was completely removed. This process yielded a dark green, soluble crude residue (Approximately 214.27 g, 6.61% w/w extraction yield).
Results
Ultraviolet-Visible (UV-Vis) spectroscopy
Figure 1 illustrates the UV-Vis spectra obtained for all experimental conditions, encompassing the control-DNA, DNA + T. aphylla, DNA + Glc + T. aphylla, and DNA + Glc. The data presented in this figure indicate that the highest absorbance value was observed for the DNA + Glc. Notably, the inclusion of T. aphylla in the Glc-containing sample resulted in a reduction of approximately 42.85% in absorbance, as depicted in Figure 1.
 Figure 1. Ultraviolet spectra of control-DNA, DNA + Tamarix aphylla, DNA + glucose + Tamarix aphylla, and DNA + glucose after 4 weeks of incubation at 37 ºC in 200 mM phosphate buffer pH 7.4 |
Figure 2 illustrates the CD spectra obtained for all experimental conditions. The control-DNA sample exhibited a negative ellipticity peak of -8 millidegrees (mdeg) at a wavelength of 255 nm and a positive ellipticity peak of +17 mdeg at 275 nm. The samples containing DNA + T. aphylla, DNA + Glc + T. aphylla, and DNA + Glc displayed negative ellipticity peaks of -6.3, -4.4, and -3.2 mdeg, respectively, all shifted to a wavelength of 245 nm. These samples also presented positive ellipticity peaks at 275 nm with magnitudes of 16.9, 12.1, and 11.9 mdeg, respectively.
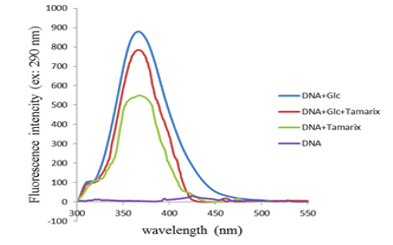 Figure 2. Fluorescence intensities of control-DNA, DNA + Tamarix aphylla, DNA + glucose + Tamarix aphylla, and DNA + glucose after 4 weeks of incubation at 37 ºC in 200 mM phosphate buffer pH 7.4 |
The fluorescence emission spectra obtained for all experimental conditions are presented in Figure 3. As illustrated therein, the highest fluorescence intensity was observed in the DNA + Glc group. These findings indicate that the presence of T. aphylla resulted in a reduction of the fluorescence emission of DNA when compared to the DNA + Glc group.
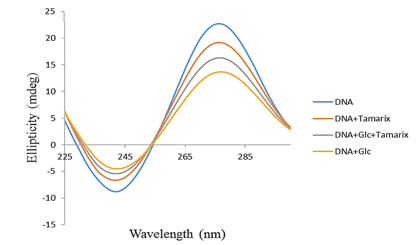 Figure 3. Circular dichroism of control-DNA, DNA + Tamarix aphylla, DNA + glucose + Tamarix aphylla, and DNA + glucose after 4 weeks of incubation at 37 ºC in 200 mM phosphate buffer pH 7.4 |
Electrophoretic analyses of all experimental groups are illustrated in Figure 4. The DNA + Glc group exhibited the highest electrophoretic mobility in comparison to other groups. Notably, the co-incubation of T. aphylla extract with DNA and Glc resulted in a substantial reduction in electrophoretic mobility, as demonstrated by the electrophoresis results presented in Figure 4.
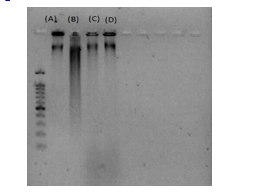 Figure 4. Agarose gel electrophoresis of native and modified DNA after 4 weeks of incubation at 37 ºC in 200 mM phosphate buffer pH 7.4: Lane (A), native DNA; Lane (B), DNA + glucose; Lane (C), DNA + Tamarix aphylla; Lane (D), DNA + glucose + Tamarix aphylla. |
Discussion
Despite ongoing efforts to elucidate the structural and functional alterations in proteins resulting from glycation, the non-enzymatic glycation of eukaryotic DNA has been the subject of comparatively limited in-depth investigation. Existing literature has documented the contribution of accumulating AGEs on both proteins and DNA to the development of diabetes mellitus and age-related pathologies (1,13). The DNA glycation process ultimately culminates in structural alterations, strand breaks, and the induction of mutations (20). Several compounds have demonstrated inhibitory effects on the glycation process, including vitamin B6 (21), aminoguanidine (22), quercetin (23), and aspirin (24). Research into agents that inhibit glycation is of significant importance for identifying their potential benefits in preventing not only the complications associated with diabetes mellitus but also certain age-related neurodegenerative disorders.
In recent scientific inquiry, there has been a significant focus on herbal medicines possessing both antiglycation and antioxidant properties as potential agents for preventing and mitigating the complications associated with the accumulation of AGEs (4). Illustratively, the seed extract of Nigella sativa has been demonstrated to inhibit protein glycation in bovine serum albumin and has also exhibited a substantial capacity for protecting against DNA damage (25). Based on the UV-Vis spectrophotometry results obtained in the present investigation, the inclusion of T. aphylla extract led to a reduction in the absorbance of DNA incubated with Glc. Prior research indicates that the UV-Vis absorbance of glycated DNA is augmented due to the partial unwinding of the double helical structure and the consequent exposure of chromophoric bases (26). Furthermore, existing literature has documented that Glc induces alterations in the biophysical and chemical characteristics of DNA (13,14). For instance, DNA exposed to Glc demonstrates hyperchromicity, a reduction in melting temperature (Tm), and an increase in emission intensity, exhibiting a temporal dependence (20). The present investigation constitutes an in vitro study that reports, for the first time, the impact of a T. aphylla extract on the structural alterations of glycated DNA. Therefore, considering the aforementioned direct effects of Glc on DNA structure, it is plausible that T. aphylla diminishes the UV-Vis absorbance of DNA through a dual mechanism involving direct interactions with the DNA molecule and indirect effects mediated by its established antioxidant and reactive oxygen species (ROS) scavenging activities. Given that ROS serve as potent mediators of cellular stress originating from the auto-oxidation of sugars (27), the antioxidant capacity of T. aphylla may contribute to the observed effects.
Based on the findings obtained from fluorescence spectroscopy, the fluorescence emission of the DNA + Glc + T. aphylla sample exhibited a reduction in intensity when compared to the DNA + Glc sample. Prior research indicates that glycated DNA has an excitation wavelength of 400 nm and an emission wavelength of 290 nm (28). Consequently, the observed decrease in fluorescence intensity suggests that the presence of T. aphylla exerts an inhibitory effect on DNA glycation and associated structural alterations. These results are in agreement with a previous investigation from our laboratory, which demonstrated the inhibitory effect of 3-β-hydroxybutyrate on the fluorescence intensity of DNA + Glc (13).
The results obtained from CD spectroscopy indicated that the negative and positive ellipticity bands in the CD spectra of the DNA + Glc exhibited an increase and a decrease in magnitude, respectively, when compared to the CD spectra of control-DNA. This observation is consistent with the findings reported in prior published research (26). Moreover, the DNA sample demonstrated fewer structural alterations when incubated in the presence of both Glc and T. aphylla extract. Consequently, the co-incubation of this plant extract with DNA and Glc appears to mitigate structural alterations and, ultimately, the formation of DNA-AGEs. These spectroscopic findings are in agreement with the results obtained from UV-Vis spectrophotometry.
Electrophoretic analysis revealed that DNA + Glc exhibited increased mobility compared to the control-DNA, a finding consistent with prior studies (13,25). Conversely, the electrophoretic mobility of DNA samples incubated with both Glc and T. aphylla was lower in this investigation. These results suggest that the presence of T. aphylla exerts an inhibitory effect on the extent of structural alterations and damage to DNA compared to DNA + Glc.
Conclusion
While ongoing research endeavors are dedicated to elucidating the structural and functional alterations in proteins resulting from glycation, the non-enzymatic glycation of eukaryotic DNA has been the subject of comparatively limited in-depth investigation. The present study offers compelling and evidence-based findings regarding the advantageous effects of T. aphylla on DNA glycation and associated structural modifications in the presence of Glc. Specifically, T. aphylla demonstrated an inhibitory influence on Glc-induced DNA structural alterations and the subsequent formation of AGEs. A reduction in the formation of AGEs was demonstrated through UV-Vis and fluorescence spectrometry. The incubation of the plant extract with DNA and Glc resulted in a decrease in UV-Vis absorbance and fluorescence intensity by approximately 33.6% and 26.6%, respectively. These observed effects appear to be mediated through both direct interactions with DNA and indirect mechanisms stemming from the established antioxidant and ROS scavenging activities of T. aphylla. Moreover, the observed effects may be attributed to the extract's potential to preserve the global conformation of the DNA molecule. This implies a possible interaction, such as stacking, with glycated nucleotides, which may direct ligands towards the glycated core. This interaction could consequently counteract the impact of inter-strand cross-links formed within the double-stranded DNA. Regardless of the precise underlying mechanisms, the findings of the current investigation warrant further in-depth studies to elucidate the detailed molecular mechanisms involved.
Acknowledgement
The authors would like to thank the staff of the central laboratory for their invaluable support in the execution of this research project.
Funding sources
This research was financially supported by a grant (Grant number: IR-UOZ-GR 9452) from the University of Zabol, Zabol, Iran.
Ethical statement
Given that this research was conducted in vitro and involved the analysis of plant DNA utilizing various biophysical methodologies, it was exempt from requiring an ethical statement.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
MB: Study design; FJHA and NP: Executing the experimental procedures; PH and MKh: Data analysis; PH and NP: Writing initial draft of the manuscript; SEB: Conducting the botanical authentication of the plant material. All listed authors have critically reviewed and approved the final version of this manuscript prior to its submission.
Data availability statement
Data can be provided upon request.
Research Article: Research Article |
Subject:
Biochemistry
Received: 2024/08/30 | Accepted: 2024/12/31 | Published: 2025/04/7 | ePublished: 2025/04/7
Received: 2024/08/30 | Accepted: 2024/12/31 | Published: 2025/04/7 | ePublished: 2025/04/7
References
1. Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, et al. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003; 27: 46616-46624. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001; 85: 746-753. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Münch G, Shepherd CE, McCann H, Brooks WS, Kwok JB, Arendt T, et al. Intraneuronal advanced glycation end products in presenilin-l Alzheimer's disease. Neuroreport 2002; 13: 601-604. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Thilavech T, Marnpae M, Mäkynen K, Adisakwattana S. Phytochemical composition, antiglycation, antioxidant activity and methylglyoxal-trapping action of brassica vegetables. Plant Foods Hum Nutr. 2021; 76; 340-346. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Qadir MI, Abbas K, Hamayun R, Ali M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L. (Saltcedar) in mice. Pak J Pharm Sci. 2014; 27(6): 1985-8. [View at Publisher] [PMID] [Google Scholar]
7. Alshehri SA, Wahab S, Abullais SS, Das G, Hani U, Ahmad W, et al. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants (Basel). 2021; 11(1): 118. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Gadallah AS, Mujeeb-Ur-Rehman, Atta-Ur-Rahman, Yousuf S, Atia-Tul-Wahab, Jabeen A, et al. Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study. Molecules. 2020; 25(13): 2994. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Mahfoudhi A, Salem AB, Garrab M, Hammami S, Gorcii M, Mastouri M, et al. Antioxidant and antimicrobial activities of Tamarix aphylla (L.) Karst. growing in Tunisia. Mor J Chem. 2016; 4(4): 987-995. [View at Publisher] [Google Scholar]
10. Qnais E, Alqudah A, Wedyan M, Athamneh RY, Bseiso Y, Abudalo R, et al. Potential anti-inflammatory activity of the Tamarix aphylla essential. Pharmacia. 2023; 70(3): 707-771. [View at Publisher] [DOI] [Google Scholar]
11. Ahmed Tariq RU, Khan N, Sharif N, Din ZU, Mansoor K. Antihyperglycemic effect of methanol extract of Tamarix aphylla L. Karst (Saltcedar) in streptozocin-nicotinamide induced diabetic rats. Asian Pacific J Tropic Biomed. 2017; 7(6): 619-623. [View at Publisher] [DOI] [Google Scholar]
12. Ashraf JM, Arif B, Dixit K, Alam K. Physicochemical analysis of structural changes in DNA modified with glucose. Int J Biol Macromol. 2012; 51: 604-611. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Bagherzadeh-Yazdi M, Bohlooli M, Khajeh M, Ghamari F, Ghaffari-Moghaddam M, Poormolaie N, et al. Acetoacetate enhancement of glucose mediated DNA glycation. Biochem Biophys Rep. 2020; 25: 100878. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Bohlooli M, Miri M, Khajeh M, et al. Inhibitory influence of 3-β-hydroxybutyrate on calf thymus DNA glycation by glucose. RSC Advances. 2016; 87: 83880-83884. [View at Publisher] [DOI] [Google Scholar]
15. Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018; 34(7): e3042. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Ali A, More TA, Hoonjan AK, Sivakami S. Antiglycating Potential of Acesulfame Potassium: An Artificial Sweetener. Appl Physiol Nutr Metab. 2017; 42: 1054-1063. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Mustafa I, Ahmad S, Dixit K, Moinuddin, Ahmad J, Ali A. Glycated human DNA is a preferred antigen for anti-DNA antibodies in diabetic patients. Diabetes Res Clin Pract. 2012; 95: 98-104. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Al-Otibi F, Moria GA, Alharbi RI, Yassin MT, Abdulaziz A, Al-Askar A. The antifungal properties of tamarix aphylla extract against some plant pathogenic fungi. Microorganisms. 2023; 11(1): 127. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine, Biochem Biophys Res Commun. 1996; 220: 113-119. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986; 232: 1629-1632. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Morimitsu Y, Yoshida K, Esaki S, Hirota A. Protein glycation inhibitors from thyme (Thymus vulgaris). Biosci Biotechnol Biochem 1995; 59: 2018-2021. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Urios P, Grigorova-Borsos AM, Sternberg M. Aspirin inhibits the formation of pentosidine, a cross-linking advanced glycation end product, in collagen, Diabetes Res Clin Pract. 2007; 77: 337-340. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Balyan P, Shamsul Ola M, Alhomida AS, Ali A. D-ribose-induced glycation and its attenuation by the aqueous extract of nigella sativa seeds. Medicina (Kaunas). 2022; 58(12): 1816. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Ahmad S, Dixit K, Shahab U, Alam K, Ali A. Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2. Biochem. Biophys. Res. Commun. 2011; 407: 568-574. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Thornalley PJ. Monosaccharide autoxidation in health and disease. Environ. Health Perspect. 1985; 64: 297-307. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Ahmad S, Shahab U, Baig MH, Khan MS, Khan MS, Srivastava AK, et al. Inhibitory effect of metformin and pyridoxamine in the formation of early, intermediate and advanced glycation end-products. PloS One. 2013; 8; e72128. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Ahmad S, Moinuddin, Shahab U, Habib S, Salman Khan M, Alam K, Ali A. Glycoxidative damage to human DNA: neo-antigenic epitopes on DNA molecule could be a possible reason for autoimmune response in type 1 diabetes. Glycobiol. 2014; 24: 281-291. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





