Volume 19, Issue 4 (Jul-Aug 2025)
mljgoums 2025, 19(4): 24-26 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tiwari N, Sharma R, Saxena P. Stressor marrow response in the case of neonatal sepsis – Significance of nRBCs in peripheral smears. mljgoums 2025; 19 (4) :24-26
URL: http://mlj.goums.ac.ir/article-1-1616-en.html
URL: http://mlj.goums.ac.ir/article-1-1616-en.html
Stressor marrow response in the case of neonatal sepsis – Significance of nRBCs in peripheral smears
1- Department of Pathology, Post Graduate Institute of Child Health, Noida, India , neema.tiwari@sharda.ac.in
2- Department of Pathology, Santosh Medical College, Ghaziabad, India
3- Department of Pathology, School of Medical Science and Research, Sharda University, Noida, India
2- Department of Pathology, Santosh Medical College, Ghaziabad, India
3- Department of Pathology, School of Medical Science and Research, Sharda University, Noida, India
Full-Text [PDF 332 kb]
(269 Downloads)
| Abstract (HTML) (937 Views)
Discussion
Sepsis is generally viewed as a disease aggravated by an inappropriate immune response (7,8). Previous studies have shown that the inflammatory response, characterized by cytokine release, is accompanied by increased nRBC production (9). Studies have found that fetuses delivered with fetal distress have normal EPO levels and elevated IL-6 concentrations (10). This implies that the fetal inflammatory response and fetal stress may have distinct roles in nRBC production and/or release into peripheral circulation. In our study, we observed that a combination of hypoxic sepsis as well as infection was a potent stimulator for the release of nRBCs in the peripheral smears.
nRBCs are normally present in fetal circulation but disappear within the first postnatal month in healthy neonates (11). nRBC counts vary by gestational and chronological age (12). In infants, nRBCs emerge from the bone marrow approximately 28 hours after a stressor such as hypoxia (13). Elevated nRBCs have been characterized as a signal of intrauterine and early postnatal stress among newborns, and they are associated with events such as fetal acidemia, meconium passage during delivery, and perinatal complications (14). In our study, we observed raised IG levels in cases where nRBCs were present in large numbers, supporting the finding that infections lead to a stress response in neonates.
nRBCs have been evaluated as a prognostic marker for outcomes following perinatal hypoxia. For infants who experience hypoxic ischemic encephalopathy, elevated nRBCs were associated with an increased risk of immediate postnatal complications, and elevated nRBC counts within the first 6 hours of life. In one study, a combination of electroencephalogram and nRBC counts demonstrated improvement over electroencephalogram alone for determining prognosis and outcomes of hypoxic ischemic encephalopathy (15).
In our case, the NLR was higher in cases with a mixed clinical picture, while the PLR was higher in cases with deranged liver functions presenting as neonatal jaundice. nRBCs were raised mostly in the mixed picture of clinical symptoms, followed by hypoxic sepsis. Compiling clinical and pathological findings together, it can be said that instead of sepsis induced by infection, the neonate’s life-threatening condition could be attributed to birth asphyxia/hypoxia; however, no documented record was available for the same.
Our finding supports the current line of thought that increased nRBC production in the immediate neonatal state primarily reflects hypoxic injury (16). Much of the existing literature focuses on hypoxia's contribution to elevated EPO levels and, thus, nRBC counts. Several studies have shown that in cases of classic chronic intrauterine fetal stress, such as preeclampsia and intrauterine growth restriction (IUGR), fetal serum and amniotic fluid EPO levels are elevated (7-12). Such a relationship has led to the prevailing idea that the nRBC count in utero, as in the adult state, is mainly driven by hypoxia-triggered EPO release.
nRBCs are present in the peripheral blood of normal infants up to the fifth day of life. They are primarily produced in the fetal bone marrow in response to erythropoietin and are stored in the marrow as precursors to reticulocytes and mature erythrocytes. In one study, the sensitivity of nRBCs for detecting sepsis was 35%, its specificity 53.48%, and its positive and negative predictive values were 23.07% and 67.64%, respectively (17). Their findings were similar to the study done by Tripathi et al. (2010) (18,19). They stated that activated macrophages release cytokines, which play an important role in stimulating nRBCs in the absence of hypoxia. She also revealed that nRBCs were significantly increased in early and late neonatal sepsis. Another study by Dulay et al. (2008) also reported a significant increase in nRBCs with early-onset neonatal sepsis (13).
In our case, we observed up to intermediate normoblasts, which are typically at the orthochromic normoblastic stage of maturation, characterized by a round nucleus with markedly dense chromatin. The cytoplasm of the circulating nucleated red cells typically displays polychromasia, and the cells are somewhat larger than mature erythrocytes, reflecting their more immature stage. The marked increase in nRBC count gives rise to a spuriously high WBC count. The appearance of nRBCs signifies bone marrow damage or stress, indicating that potentially serious underlying disease and extramedullary hematopoiesis have been activated.
In this case, we did not calculate the standard CBC indicators of neonatal sepsis, since our primary findings were of nRBCs and late and intermediate normoblasts in the smear examined.
Abbreviations
NLR: Neutrophil Lymphocyte Ratio; LMR: Lymphocyte Monocyte Ratio; IG: Immature Granulocyte; nRBC: nucleated Red Blood Cells; TLC: Total Leucocyte Count; PLR: Platelet Lymphocyte Ratio
Conclusion
We conclude that such an extreme manifestation of neonatal sepsis, with the main response being erythroid lineage spillover in peripheral blood, is rare and can be used as a soft indicator for the etiology of the disease to be interpreted.
Acknowledgement
We appreciate the Department of Pathology, PGICH, Noida; Santosh Ghaziabad; and SMSR, Greater Noida.
Funding sources
Not applicable.
Ethical statement
The research study has been approved by the institutional ethics committee and is compliant with all necessary regulations. Approval for a waiver from the institutional ethics committee has been obtained (Ethical Code: SMSR/IEC/274 and applied for at PGICH, Noida).
Conflicts of interest
All contributing authors declare no conflicts of interest.
Author contributions
All contributing authors declare no conflicts of interest.
Data availability statement
The data has been sourced from the archived records of the Pathology department at the specified medical colleges.
Full-Text: (132 Views)
Introduction
Neonatal sepsis is a life-threatening condition caused by systemic bacterial, viral, or fungal infection within the first 28 days of life. It is associated with hemodynamic changes, clinical manifestations, and results in neonatal morbidity and mortality. Nucleated red blood cells (nRBCs) are immature erythrocytes whose production is thought to be driven primarily by the interplay of hypoxia and erythropoietin (EPO) synthesis (1,2). The clinical presentation of neonatal sepsis is non-specific and may include fever, refusal to feed, respiratory distress, lethargy, irritability, convulsions, bulging fontanels, abdominal distension, and temperature dysregulation. Neonatal sepsis is classified as early-onset (Occurring within the first 24 hours of life) or late-onset (Occurring after 48-72 hours) (3,4).
Multiple hematological and biochemical markers are used in diagnosing neonatal sepsis. Defined hemogram criteria exist for diagnosis. Some of the indicators established or under study include total leukocyte count (TLC), absolute neutrophil count (ANC), immature neutrophil count, immature-to-total neutrophil (I/T) ratio, platelet count (PLT), mean platelet volume (MPV), and platelet distribution width (PDW). A CBC and differential leukocyte count may help detect infection-associated changes (e.g., thrombocytopenia or neutropenia) or monitor a left shift in neutrophils. However, the sensitivity and specificity of these markers are low (5,6). Serial monitoring of the CBC may help differentiate sepsis from non-specific abnormalities due to delivery stress (2,3).
The presence of nRBCs in peripheral smears of neonatal sepsis cases indicates a marrow stress response. This study presents a series of neonates with original research data highlighting the presence of nRBCs in neonatal sepsis.
Methods
Retrospective analysis of 27 CBC and peripheral blood smears of neonates was done over one month, where peripheral smears had been made and stored for examination, and reports for the peripheral smears had been dispatched. Clinical details (As available from the clinician), parameters for neonatal sepsis (NLR, PLR, IG), platelet counts, and nRBC counts were recorded and tabulated. For result tabulation, we divided the neonatal samples into two groups: 0-1 day and 2-30 days.
Results
Retrospective data with clinical history were collected from records available in the Department of Pathology archives, as well as from archives in the medical records department for clinical details. Data were collected for all cases having nRBCs in CBC and peripheral smears exceeding a count of 10 nRBCs/100 WBCs. NLR and PLR were calculated from CBC, and IG was calculated from data available in CBC and peripheral smears.
The study analyzed 26 cases, of which 17 were males and 9 were females; all neonates were between 0 and 30 days of age. Demographic details of the cases were recorded. Table 1 highlights the age-related findings (As per days) of CBC parameters under study in neonates 0-1 day and 2-30 days. An average of individual parameters was calculated, and it was noted that in the 0–1 day group, an average of 11.6 nRBCs was observed in smears, while 39.6 nRBCs were observed in neonates aged 2-30 days.
The table indicated the average values of CBC-derived parameters, as well as the percentage of IGs divided according to patient age. This was a descriptive analysis highlighting the average values of parameters in the study.
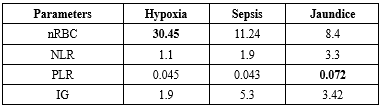
Table 2 shows the correlation of pathological parameters, where the numbers highlight the average percentage of cases. For example, 30.45% of cases with hypoxia showed nRBCs, 11.24% with sepsis showed nRBCs, 8.4% with neonatal jaundice showed nRBCs, and 50.08% of cases had a combination of clinical presentations such as jaundice with sepsis and hypoxic sepsis, and showed nRBCs in the smears.
A significant NLR ratio (6.3) was seen in cases where sepsis, hypoxia, or jaundice co-existed, as compared to single entities like hypoxia, sepsis, or jaundice.
The PLR ratio was significant in cases with jaundice with nRBCs in the smear as compared to other cases. IGs were seen more (10.62) in cases with mixed clinical features.
Neonatal sepsis is a life-threatening condition caused by systemic bacterial, viral, or fungal infection within the first 28 days of life. It is associated with hemodynamic changes, clinical manifestations, and results in neonatal morbidity and mortality. Nucleated red blood cells (nRBCs) are immature erythrocytes whose production is thought to be driven primarily by the interplay of hypoxia and erythropoietin (EPO) synthesis (1,2). The clinical presentation of neonatal sepsis is non-specific and may include fever, refusal to feed, respiratory distress, lethargy, irritability, convulsions, bulging fontanels, abdominal distension, and temperature dysregulation. Neonatal sepsis is classified as early-onset (Occurring within the first 24 hours of life) or late-onset (Occurring after 48-72 hours) (3,4).
Multiple hematological and biochemical markers are used in diagnosing neonatal sepsis. Defined hemogram criteria exist for diagnosis. Some of the indicators established or under study include total leukocyte count (TLC), absolute neutrophil count (ANC), immature neutrophil count, immature-to-total neutrophil (I/T) ratio, platelet count (PLT), mean platelet volume (MPV), and platelet distribution width (PDW). A CBC and differential leukocyte count may help detect infection-associated changes (e.g., thrombocytopenia or neutropenia) or monitor a left shift in neutrophils. However, the sensitivity and specificity of these markers are low (5,6). Serial monitoring of the CBC may help differentiate sepsis from non-specific abnormalities due to delivery stress (2,3).
The presence of nRBCs in peripheral smears of neonatal sepsis cases indicates a marrow stress response. This study presents a series of neonates with original research data highlighting the presence of nRBCs in neonatal sepsis.
Methods
Retrospective analysis of 27 CBC and peripheral blood smears of neonates was done over one month, where peripheral smears had been made and stored for examination, and reports for the peripheral smears had been dispatched. Clinical details (As available from the clinician), parameters for neonatal sepsis (NLR, PLR, IG), platelet counts, and nRBC counts were recorded and tabulated. For result tabulation, we divided the neonatal samples into two groups: 0-1 day and 2-30 days.
Results
Retrospective data with clinical history were collected from records available in the Department of Pathology archives, as well as from archives in the medical records department for clinical details. Data were collected for all cases having nRBCs in CBC and peripheral smears exceeding a count of 10 nRBCs/100 WBCs. NLR and PLR were calculated from CBC, and IG was calculated from data available in CBC and peripheral smears.
The study analyzed 26 cases, of which 17 were males and 9 were females; all neonates were between 0 and 30 days of age. Demographic details of the cases were recorded. Table 1 highlights the age-related findings (As per days) of CBC parameters under study in neonates 0-1 day and 2-30 days. An average of individual parameters was calculated, and it was noted that in the 0–1 day group, an average of 11.6 nRBCs was observed in smears, while 39.6 nRBCs were observed in neonates aged 2-30 days.
The table indicated the average values of CBC-derived parameters, as well as the percentage of IGs divided according to patient age. This was a descriptive analysis highlighting the average values of parameters in the study.
Table 2 shows the correlation of pathological parameters, where the numbers highlight the average percentage of cases. For example, 30.45% of cases with hypoxia showed nRBCs, 11.24% with sepsis showed nRBCs, 8.4% with neonatal jaundice showed nRBCs, and 50.08% of cases had a combination of clinical presentations such as jaundice with sepsis and hypoxic sepsis, and showed nRBCs in the smears.
A significant NLR ratio (6.3) was seen in cases where sepsis, hypoxia, or jaundice co-existed, as compared to single entities like hypoxia, sepsis, or jaundice.
The PLR ratio was significant in cases with jaundice with nRBCs in the smear as compared to other cases. IGs were seen more (10.62) in cases with mixed clinical features.
Discussion
Sepsis is generally viewed as a disease aggravated by an inappropriate immune response (7,8). Previous studies have shown that the inflammatory response, characterized by cytokine release, is accompanied by increased nRBC production (9). Studies have found that fetuses delivered with fetal distress have normal EPO levels and elevated IL-6 concentrations (10). This implies that the fetal inflammatory response and fetal stress may have distinct roles in nRBC production and/or release into peripheral circulation. In our study, we observed that a combination of hypoxic sepsis as well as infection was a potent stimulator for the release of nRBCs in the peripheral smears.
nRBCs are normally present in fetal circulation but disappear within the first postnatal month in healthy neonates (11). nRBC counts vary by gestational and chronological age (12). In infants, nRBCs emerge from the bone marrow approximately 28 hours after a stressor such as hypoxia (13). Elevated nRBCs have been characterized as a signal of intrauterine and early postnatal stress among newborns, and they are associated with events such as fetal acidemia, meconium passage during delivery, and perinatal complications (14). In our study, we observed raised IG levels in cases where nRBCs were present in large numbers, supporting the finding that infections lead to a stress response in neonates.
nRBCs have been evaluated as a prognostic marker for outcomes following perinatal hypoxia. For infants who experience hypoxic ischemic encephalopathy, elevated nRBCs were associated with an increased risk of immediate postnatal complications, and elevated nRBC counts within the first 6 hours of life. In one study, a combination of electroencephalogram and nRBC counts demonstrated improvement over electroencephalogram alone for determining prognosis and outcomes of hypoxic ischemic encephalopathy (15).
In our case, the NLR was higher in cases with a mixed clinical picture, while the PLR was higher in cases with deranged liver functions presenting as neonatal jaundice. nRBCs were raised mostly in the mixed picture of clinical symptoms, followed by hypoxic sepsis. Compiling clinical and pathological findings together, it can be said that instead of sepsis induced by infection, the neonate’s life-threatening condition could be attributed to birth asphyxia/hypoxia; however, no documented record was available for the same.
Our finding supports the current line of thought that increased nRBC production in the immediate neonatal state primarily reflects hypoxic injury (16). Much of the existing literature focuses on hypoxia's contribution to elevated EPO levels and, thus, nRBC counts. Several studies have shown that in cases of classic chronic intrauterine fetal stress, such as preeclampsia and intrauterine growth restriction (IUGR), fetal serum and amniotic fluid EPO levels are elevated (7-12). Such a relationship has led to the prevailing idea that the nRBC count in utero, as in the adult state, is mainly driven by hypoxia-triggered EPO release.
nRBCs are present in the peripheral blood of normal infants up to the fifth day of life. They are primarily produced in the fetal bone marrow in response to erythropoietin and are stored in the marrow as precursors to reticulocytes and mature erythrocytes. In one study, the sensitivity of nRBCs for detecting sepsis was 35%, its specificity 53.48%, and its positive and negative predictive values were 23.07% and 67.64%, respectively (17). Their findings were similar to the study done by Tripathi et al. (2010) (18,19). They stated that activated macrophages release cytokines, which play an important role in stimulating nRBCs in the absence of hypoxia. She also revealed that nRBCs were significantly increased in early and late neonatal sepsis. Another study by Dulay et al. (2008) also reported a significant increase in nRBCs with early-onset neonatal sepsis (13).
In our case, we observed up to intermediate normoblasts, which are typically at the orthochromic normoblastic stage of maturation, characterized by a round nucleus with markedly dense chromatin. The cytoplasm of the circulating nucleated red cells typically displays polychromasia, and the cells are somewhat larger than mature erythrocytes, reflecting their more immature stage. The marked increase in nRBC count gives rise to a spuriously high WBC count. The appearance of nRBCs signifies bone marrow damage or stress, indicating that potentially serious underlying disease and extramedullary hematopoiesis have been activated.
In this case, we did not calculate the standard CBC indicators of neonatal sepsis, since our primary findings were of nRBCs and late and intermediate normoblasts in the smear examined.
Abbreviations
NLR: Neutrophil Lymphocyte Ratio; LMR: Lymphocyte Monocyte Ratio; IG: Immature Granulocyte; nRBC: nucleated Red Blood Cells; TLC: Total Leucocyte Count; PLR: Platelet Lymphocyte Ratio
Conclusion
We conclude that such an extreme manifestation of neonatal sepsis, with the main response being erythroid lineage spillover in peripheral blood, is rare and can be used as a soft indicator for the etiology of the disease to be interpreted.
Acknowledgement
We appreciate the Department of Pathology, PGICH, Noida; Santosh Ghaziabad; and SMSR, Greater Noida.
Funding sources
Not applicable.
Ethical statement
The research study has been approved by the institutional ethics committee and is compliant with all necessary regulations. Approval for a waiver from the institutional ethics committee has been obtained (Ethical Code: SMSR/IEC/274 and applied for at PGICH, Noida).
Conflicts of interest
All contributing authors declare no conflicts of interest.
Author contributions
All contributing authors declare no conflicts of interest.
Data availability statement
The data has been sourced from the archived records of the Pathology department at the specified medical colleges.
Research Article: Brief Report |
Subject:
Others
Received: 2023/01/26 | Accepted: 2024/06/1 | Published: 2025/09/17 | ePublished: 2025/09/17
Received: 2023/01/26 | Accepted: 2024/06/1 | Published: 2025/09/17 | ePublished: 2025/09/17
References
1. Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001;84(3):F211-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Christensen RD, Henry E, Andres RL, Bennett ST. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology 2011;99(4):289-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Christensen RD, Lambert DK, Richards DS. Estimating the nucleated red blood cell "emergence time" in neonates. J Perinatol. 2014;34(2):116-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Hanlon-Lundberg KM, Kirby RS. Nucleated red blood cells as a marker of acidemia in term neonates. Am J Obstet Gynecol. 1999;181(1):196-201. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Kovalak EE, Dede FS, Gelisen O, Dede H, Haberal A. Non reassuring fetal heart rate patterns and nucleated red blood cells in term neonates. Arch Gynecol Obstet. 2011;283(5):1005-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Baschat AA, Gembruch U, Reiss I, Gortner L, Harman CR. Neonatal nucleated red blood cell count and postpartum complications in growth restricted fetuses. J Perinat Med. 2003;31:323e9. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Boskabadi H, Zakerihamidi M, Sadeghian MH, Avan A, Ghayour Mobarhan M, Ferns GA. Nucleated red blood cells count as a prognostic biomarker in predicting the complications of asphyxia in neonates. J Matern Fetal Neonatal Med. 2017;30(21):2551-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Li J, Kobata K, Kamei Y, Okazaki Y, Nishihara M, Wada H, et al. Nucleated red blood cell counts: an early predictor of brain injury and 2-year outcome in neonates with hypoxic-ischemic encephalopathy in the era of cooling-based treatment. Brain Dev .2014;36(6):472-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Walsh BH, Boylan GB, Murray DM. Nucleated red blood cells and early EEG: predicting Sarnat stage and two year outcome. Early Hum Dev. 2011;87(5):335-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Silva AM, Smith RN, Lehmann CU, Johnson EA, Holcroft CJ, Graham EM. Neonatal nucleated red blood cells and the prediction of cerebral white matter injury in preterm infants. Obstet Gynecol. 2006;107(3):550-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Mandel D, Lubetzky R, Mimouni FB, Cohen S, Littner Y, Deutsch V, et al. Nucleated red blood cells in preterm infants who have necrotizing enterocolitis. J Pediatr. 2004;144(5):653-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Bin-Nun A, Mimouni FB, Fink D, Sela H, Hammerman C. Elevated nucleated red blood cells at birth predict hemodynamically significant patent ductus arteriosus. J Pediatr. 2016;177:313-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with earlyonset neonatal sepsis. Am J Obstet Gynecol. 2008;198(4):426.e1-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Cremer M, Roll S, Graf C, Weimann A, Bu¨hrer C, Dame C. Nucleated red blood cells as marker for an increased risk of unfavorable outcome and mortality in very low birth weight infants. Early Hum Dev. 2015;91(10):559-63. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Morton S, Bretten K, Feldman HA, Leeman KT. Association of nucleated red blood cell count with mortality among neonatal intensive care unit patients. Pediatr Neonatol. 2020:61(6);592-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Xanthou. Leucocyte blood picture in ill newborn babies. Arch Dis Child. 1972;47(255):741-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Abhishek MG,Sanjay M. Diagnostic efficacy Nrbc count in early diagnosis of neonatal sepsis. IJPO. 2015;2(4);182-5. [View at Publisher] [DOI]
18. Tripathi S, Malik GK. Neonatal sepsis: past, present and future; a review article. Internet Journal of Medical Update. 2010;5(2):45-54. [View at Publisher] [DOI] [Google Scholar]
19. Purtle SW, Horkan CM, Moromizato T, Gibbons FK, Christopher KB. Nucleated red blood cells, critical illness survivors and post-discharge outcomes: a cohort study. Crit Care. 2017;21(1):154. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.