Volume 10, Issue 4 (12-2022)
Jorjani Biomed J 2022, 10(4): 60-69 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemi Shiri Z, Bagherpour T, nemati N. Aerobic Training and Curcumin can Improve Adiponectin Gene Expression in Adipose Tissue and Body Mass Index of Rats Fed with High-Fat Diet. Jorjani Biomed J 2022; 10 (4) :60-69
URL: http://goums.ac.ir/jorjanijournal/article-1-928-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-928-en.html
1- Department of Sports Physiology, Damghan Branch, Islamic Azad University Damghan, Damghan, I.R. Iran.
2- Department of Sports Physiology, Damghan Branch, Islamic Azad University Damghan, Damghan, I.R. Iran. ,bagherpour@damghaniau.ac.ir
3- Department of Sports Physiology, Damghan Branch, Islamic Azad University Damghan, Damghan, I.R
2- Department of Sports Physiology, Damghan Branch, Islamic Azad University Damghan, Damghan, I.R. Iran. ,
3- Department of Sports Physiology, Damghan Branch, Islamic Azad University Damghan, Damghan, I.R
Keywords: Exercise [MeSH], Curcumin [MeSH], Adiponectin [MeSH], Body Mass Index [MeSH], Diet, High-Fat [MeSH]
Full-Text [PDF 437 kb]
(3190 Downloads)
| Abstract (HTML) (9824 Views)
Full-Text: (1755 Views)
Introduction
Today, the prevalence of obesity in eastern and developed countries and even developing countries has increased due to lifestyle. To be more specific, this prevalence has doubled from 1980 to 2015 in more than 70 countries, and has caused health problems for these people (1). Reduced physical activity together with not following a proper diet in individuals causes a disturbance in metabolism and storage of extra calories as white and visceral fat tissue, resulting in malfunctions of various organs such as the heart, liver, muscle as well as disturbance in fat tissue metabolism (2). In addition, increased intake of calories compared to the reduced calories received through physical activity is associated with an increase in visceral fat mass, and this is associated with a decrease in physical performance and an increase in cardiovascular risks (3). Researchers suggest that endocrine disturbances in visceral fat tissue are the first stage of cardiovascular diseases following obesity. In other words, disturbance in hormones derived from adipose tissue such as adiponectin is the most important factor for disturbance in metabolism (4).
Adiponectin has anti-inflammatory effects and increases insulin sensitivity (4). Researchers are looking for the best and fastest way to adjust the calories consumed and taken in, so that by reducing the fat weight, the death rate caused by such diseases can be reduced (3). It seems that exercise training with the mechanism of increasing the activation of hormone-sensitive lipase, increasing the release of triglycerides and transferring them into mitochondria for metabolism can lead to a decrease in visceral fat weight (5). Regular exercise can inhibit pro-inflammatory factors by increasing the release of adiponectin from fat tissue and have beneficial effects on physical health (6). Studies show that various types of training (high intensity interval traini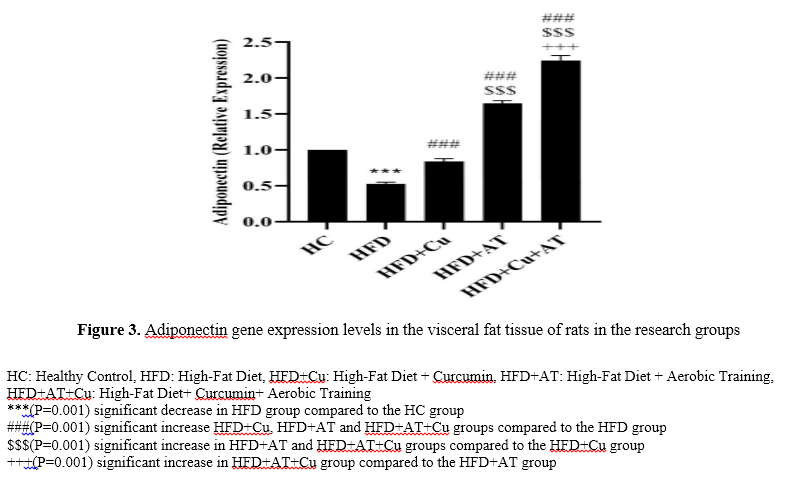 ng and low intensity continuous training) reduce calorie intake, reduce visceral fat weight and improve aerobic capacity in animal models fattened by high-fat food and suffering from diabetes (3). Also, long-term aerobic exercise training together with controlling calorie intake increased adiponectin to leptin ratio in overweight elderly (4). In Ouerghi et al.'s study, the results showed that although high intensity interval training improved insulin sensitivity and reduced inflammatory factors, no significant change was observed in adiponectin and omentin levels in overweight healthy young men (7).
ng and low intensity continuous training) reduce calorie intake, reduce visceral fat weight and improve aerobic capacity in animal models fattened by high-fat food and suffering from diabetes (3). Also, long-term aerobic exercise training together with controlling calorie intake increased adiponectin to leptin ratio in overweight elderly (4). In Ouerghi et al.'s study, the results showed that although high intensity interval training improved insulin sensitivity and reduced inflammatory factors, no significant change was observed in adiponectin and omentin levels in overweight healthy young men (7).
On the other hand, considering the conflicting results regarding the effect of training caused by the duration, intensity and type of training, researchers have suggested the use of a proper diet and medicinal plants in addition to exercise training for greater efficiency (8, 9). Among the natural products, Curcumin (Cu), which is the most important component of turmeric, has been used in the treatment of many diseases for a long time, consequently this polyphenol has favorable biological effects such as anti-cancer, anti-diabetes, lowering blood sugar, improving liver enzymes, and improving some symptoms of metabolic syndrome (10). Also, researchers have suggested that the consumption of Cu can suppress pro-inflammatory and inflammatory cytokines in fat tissue and prevent the occurrence of some diseases from this pathway (11). Regarding the effect of Cu on fat tissue metabolism, researchers in a meta-analysis study on clinical trials showed that this natural antioxidant reduces Body Mass Index (BMI), decreases weight, and increases adiponectin in metabolic syndrome and related diseases (12). In addition, in a review study, some other researchers showed that the consumption of Cu leads to the inhibition of inflammatory factors in fat tissue and is also known as an anti-obesity factor (13).
Considering the conflicting results regarding the type and intensity-dependent effects of training on adiponectin changes, as well as the presence of an effective anti-obesity substance without side effects such as Cu, it seems that few studies have addressed the simultaneous mechanism of the use of Aerobic Training (AT) and Cu consumption in visceral fat tissue, and thus investigating the simultaneous effect of these two interventions can provide more comprehensive information for obese and overweight individuals to lose weight and reach fitness. Given the limited information in this regard, the aim of this study was to investigate the effect of six weeks of AT and Cu consumption on adiponectin gene expression levels in the visceral adipose tissue and some anthropometric characteristics of rats fed with a High-Fat Diet (HFD).
Materials and Methods
Preparation and maintenance of animals
In this experimental and fundamental study, 50 male rats with an age range of 8-10 weeks and a weight range of 180-220 grams were prepared from the breeding and reproduction center of Islamic Azad University of Zanjan branch. Throughout the research period, the ethical principles of working with animals were conducted based on the Helsinki Agreement and under the supervision of the ethics committee of Shahrood University of Medical Sciences with the approved code IR.SHMU.REC. 2018.87.
It is worth noting that all over the research period, the animals were kept under standard conditions in terms of light (12:12 hours of light and darkness), temperature (22-24°C), and relative humidity (55-60%). The animals were kept in washable polycarbonate cages and had ad libitum access to water and special food for rats. Also, sterile grated wood was used to absorb moisture and urine on the bottom of the cages. the rats were randomly divided into five groups, including: (1) healthy control (HC), (2) High-Fat Diet control (HFD), (3) consumption of curcumin + high-fat diet (HFD+Cu), (4) aerobic training + high-fat diet (HFD+AT), and (5) aerobic training + curcumin + high-fat diet (HFD + Cu + AT).
Preparation of high-fat diet (HFD)
In this research, high-fat diet emulsion was prepared so that each kilogram of it contained 400 grams of corn oil, 150 grams of sucrose, 80 grams of whole milk powder, 100 grams of cholesterol, 2.5 grams of multivitamins, 36.5 grams of Tween 80, 31 grams of propylene glycol and 10 grams of salt. Food and water were freely available for all rats, however the high-fat diet groups were given 1.5 mg/kg of body weight of this emulsion each day by gavage (14).
Aerobic training protocol
After grouping and adaptation, the rats were initially introduced to the laboratory animal treadmill. Habituation took place daily for 10 minutes at a speed of 8 m/min for one week. Also, to calculate the maximum running speed, which is equivalent to the maximum oxygen consumption in rats based on studies, the protocol of Natio et al. (2001) was used. Next, the relative intensity of endurance training in this research was considered equal to 75% of the maximum oxygen consumption; for this purpose, the incline of the treadmill at the beginning of the training period was considered 15%. Also, the AT program in this research started with a speed of 25 m/min for 10 minutes and reached 30 m/min for 50 minutes in the sixth week. In addition, rats in this research ran for 5 minutes at a speed of 10-15 m/min on a zero-degree slope at the beginning and end of the training to warm up and cool down (15). A better description of the training protocol is presented in Table 1 (15).

Curcumin consumption
In this study, rats received 1/5 g/kg daily by gavage. To this end, the Cu manufactured by Sigma-Aldrich (CAS Number: 458-37-7) was ordered to be prepared (16).
Dissection and sampling
After the end of the course and 48 hours after the last training session, rats fasted for 12 hours using a desiccator, and were anesthetized with a combination of 90 mg/kg ketamine and 10 mg/kg xylazine. After completing anesthesia and making sure of unconsciousness through tests of pain sensation and foot pressing and observing no pain reflex, the weight of the rats was initially measured using a digital scale with an accuracy of 0.01 grams, then the abdominal cavity of the rats was split with a scalpel and after discarding the excess tissues, all the visceral fat tissues, including the fat tissues around the intestines, the abdominal cavity and the fat tissues attached to the sides and back of the rats, were carefully extracted. The weight of all visceral adipose tissues was measured using a digital scale and then the tissues were placed in special tissue storage microtubes containing RNA Later. It is worth mentioning that the samples were immediately placed at -70°C and kept for the next steps.
Adiponectin gene expression measurement method
To measure adiponectin gene expression levels, the visceral fat tissue sample was first homogenized, then RNA extraction was performed using the recommended protocol in the special RNA extraction kit called plus-RNX manufactured by Sina Gene Company. To ensure the percentage of RNA purity, a spectrophotometer was used at wavelengths of 200 to 500 nm. After extracting and ensuring the purity of RNA, the samples were prepared for cDNA synthesis. For this purpose, the protocol recommended in the cDNA synthesis kit made by Takara Company in Japan was used. Next, the NCBI site was used to design leptin and adiponectin primers, and the primers were designed based on the guide available on this site. Also, to ensure the efficiency of the designed primers, the online software available on the NCBI website was used. After determining the efficiency of the primers, the reverse transcription reaction was performed using a Real Time-PCR device. Also, internal control gene Beta-2-Myoglobulin (B2M) was used to quantify the data, and after the completion, the formula 2-ΔΔCt was used to quantify the data according to the expression threshold cycle of the samples.
Data analysis procedure
In this research, the mean and standard deviation were first reported in the descriptive statistics, and then the Shapiro-Wilk test was used to check the normality of the data distribution. Next, to check the difference between groups in the BMI variables, one-way analysis of variance was used along with Tukey's post hoc test. Also, to analyze the pre-test and post-test data of the weight variable, and to remove the pre-test effect, covariance test and Bonferroni's post hoc test were used. In addition, the data of this research were analyzed using GraphPad PRISM 3.8.3 software at a significance level of P≤0.05.
Results
The results of one-way analysis of variance showed a significant difference in weight (P=0.001 and F=53.48), BMI (P=0.003 and F=4.61) and adiponectin gene expression (P=0.001 and F=5.60) in the research groups.
The results of the Bonferroni's post hoc test showed that the post-test weight levels in the HFD group were significantly higher than the HC group (P=0.001), however in the HFD+Cu (P=0.001), HFD+AT (P=0.001) and HFD+AT+Cu (P=0.001) groups, the levels were significantly lower than the HFD group. The levels were also significantly lower in the HFD+AT (P=0.001) and HFD+AT+Cu (P=0.001) groups than the HFD+Cu group. In addition, the levels were significantly lower in the HFD+AT+Cu group than the HFD+AT group (P=0.011) (Figure 1).
.PNG)
The results of Tukey's post hoc test showed that there was no significant difference in BMI levels in the HFD and HC groups (P=0.39). However, in the HFD+AT (P=0.02) and HFD+AT+Cu (P=0.008) groups, the levels were significantly lower than the HFD group. The levels were also significantly lower in the HFD+AT+Cu group than the HFD+Cu group (P=0.04) (Figure 2).
.PNG)
Adiponectin levels in the HFD group were significantly lower than the HC group (P=0.001). But in the HFD+Cu (P=0.001), HFD+AT (P=0.001) and HFD+Cu+AT (P=0.001) groups, the levels were significantly higher than the HFD group. The levels were also significantly higher in the HFD+AT (P=0.001) and HFD+Cu+AT (P=0.001) groups than the HFD+Cu group. In addition, the levels were significantly higher in the HFD+Cu+AT group than the HFD+AT group (P=0.001) (Figure 3).
.PNG)
Discussion
The results of the present study showed that the gene expression levels of adiponectin decreased following the high-fat diet, while the weight increased. On the other hand, AT increased the expression of adiponectin, while it decreased weight and BMI of rats exposed to high-fat diet. According to the studies conducted, the main cause of metabolic diseases following weight gain and obesity is the increase in insulin resistance. That is, an increase in insulin resistance exposes the cell to increased free radicals and damage with metabolic disorder. In addition, the increase in insulin resistance is associated with a decrease in the amount of adiponectin in adipose tissue, and simultaneously, the amount of leptin increases, which has an inverse relationship with adiponectin (17).
In addition, researchers have suggested that leptin increases following weight gain, and since it has a role in metabolic regulation, due to the destruction of its receptors on the cell surface, this hormone does not bind to its receptors properly, and as a result, a leptin resistance occurs making its function inadequate, and this activates T cells and stimulates the immune system. Subsequently, the stimulation of the immune system leads to an increase in the expression of inflammatory cytokines; on the other hand, adiponectin from AMP-Activated Protein Kinase (AMPK) leads to increased lipolysis and is directly associated with fat weight loss (18). Nonetheless, by increasing the metabolic demand to provide energy for cell life, exercise acts as a secretion regulator of leptin and adiponectin hormones; in other words, exercise activates hormone-sensitive lipase by phosphorylating protein kinases and leads to increased lipolysis in adipose tissue, thereby increasing the catabolism of fats in cells that need energy. As the diameter of fat vacuoles decreases in adipose cells, insulin sensitivity increases, and this is associated with a decrease in leptin expression and leptin resistance in other cells, and since there is an inverse relationship between adiponectin and leptin, an increase in nuclear transcription proteins leads to an increase in the gene expression and transcription of adiponectin (19). In line with the present study, in a review and meta-analysis, researchers stated that exercise increased adiponectin in 2996 people (20). Also, 20 weeks of caloric restriction training and exercise led to weight loss, reduction of body fat percentage and increase of plasma adiponectin in obese women (21). On the other hand, in a study, researchers showed that although 12 weeks of combined training improved BMI, reduced body fat percentage, increased net body mass and improved glucose metabolism, it did not have a significant effect on adiponectin levels in obese children (22). Banaii Broojeni et al showed that a progressive resistance-training program could prevent the loss of lean body mass and improve the reduction of total and abdominal fat mass and insulin resistance in overweight adult men, but it had no significant effect on adiponectin (23). It seems that the frequency and type of training were difference between our study and the above studies. Because the training performed in Jeon et al.'s study comprised two sessions per week, but in our study, they comprised five sessions per week (22). Also, in another study, the researchers stated that 6 months of AT, four sessions a week and each session 45 minutes with an intensity of 65 to 80% of the peak oxygen consumption increased insulin sensitivity, however there was no significant difference between adiponectin levels and body fat percentage in the training and control groups (24). These researchers concluded that exercise training depending on the amount of the effect on fat mass can change adiponectin levels. Therefore, it seems that changes in adiponectin levels following exercise are dependent on the changes in the fat percentage and its lipolysis.
The results of the present study showed that the consumption of Cu increased the expression of adiponectin and decreased the weight of rats exposed to high-fat diet. Based on the studies conducted, it seems that Cu by upregulating mechanism of AMP-Activated Protein Kinase (AMPK) and by activating Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) co-activator, increasing hepatic protein kinase B, activating deacylation-dependent proteins such as sirtuin 1 and 3 (SIRT1/3), leads to a decrease in inflammatory cytokines through affecting monocyte chemoattractant protein-1, increasing lipolysis, and increasing mitochondrial biogenesis, and results in an increase in adiponectin expression , decrease in fat weight and finally increase in lipolysis (12). In their study, Akbari et al stated that Cu consumption led to a decrease in BMI, waist to hip circumference ratio, body fat percentage, and leptin levels, and an increase in adiponectin in children with metabolic syndrome and related diseases (12). It was also stated in Bradford's study that Cu consumption had a beneficial effect on obesity and related diseases through lipolytic and anti-inflammatory biological activities (13). Also, consistent with the findings of this study, in a review study, the researchers stated that the consumption of Culed to an increase in adiponectin levels (25). Also, the results showed that simultaneous AT and Cu consumption increased the expression of adiponectin and decreased the weight and BMI of rats exposed to high-fat diet. In addition, the effect of training on the increase of adiponectin and decrease of weight and BMI was higher than Cu consumption.
According to the studies, it seems that both exercise training and Cu consumption through similar mechanisms of AMPK activation, PPAR-γ and SIRT1/3 phosphorylation (12, 18), reduction of inflammatory factors and improvement of immune system function (13,19) lead to increased expression of adiponectin. In addition, it seems that frequency-dependent exercise training and the basic levels of body fat percentage can affect adiponectin levels. On the other hand, the use of Cu in longer periods and higher doses has a more favorable effect on the metabolism of fat tissue. Regarding the simultaneous effect of training and Cu consumption, researchers have shown that IL-10 and Brain-Derived Neurotrophic Factor (BDNF) levels increased and IL-6 levels decreased significantly. However, the antioxidant effect of Cu was greater than training so that it caused a greater decrease in MDA and C-reactive protein in women with metabolic syndrome (26). In another study researchers showed that Swimming and curcumin consumption simultaneously can significantly moderate apoptosis caused by ethanol in the heart tissue (27). Also, in a study, combined training along with Cu consumption increased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (PGC1-α), Forkhead box protein O1 (FOXO1), and decreased cardiac tissue atrophying protein in obese Wistar rats (28). Likewise, in another study, researchers showed that combined training and Cu consumption improved fat metabolism in inactive middle-aged men (29). Therefore, it seems that exercise training and Cu consumption can be synergistically effective in increasing lipolysis, reducing body fat percentage, and increasing adiponectin.
Due to the effect of the frequency and intensity of exercise training and the affectability of the body's oxidation system to these factors, the lack of measurement in this study was one of the limitations of our study. Therefore, it is suggested to evaluate oxidant-antioxidant markers in future studies along with different exercise intensities. Also, the functional complexity of cytokines and adipokines derived from adipose tissue and the lack of measurement of some of them, such as leptin, are other limitations of the present study. Thus, it is suggested to evaluate leptin levels along with histological examination and the size of adipose tissue cells in the following studies.
Conclusion
It seems that curcumin consumption and exercise training both individually and synergistically can lead to weight loss and improve body mass index by improving adiponectin. Therefore, the use of these two (aerobic training and curcumin) is suggested in the conditions of metabolic disorders.
Acknowledgment
This research was conducted in the form of doctoral thesis of Ms. Zahra Hashemi Shiri at Islamic Azad University, Semnan branch. The authors hereby express their gratitude to the vice president of research of this university.
Funding source(s)
The researchers did not receive any financial support for this research.
Conflict of interest
The authors declare that there is no conflict of interest.
Today, the prevalence of obesity in eastern and developed countries and even developing countries has increased due to lifestyle. To be more specific, this prevalence has doubled from 1980 to 2015 in more than 70 countries, and has caused health problems for these people (1). Reduced physical activity together with not following a proper diet in individuals causes a disturbance in metabolism and storage of extra calories as white and visceral fat tissue, resulting in malfunctions of various organs such as the heart, liver, muscle as well as disturbance in fat tissue metabolism (2). In addition, increased intake of calories compared to the reduced calories received through physical activity is associated with an increase in visceral fat mass, and this is associated with a decrease in physical performance and an increase in cardiovascular risks (3). Researchers suggest that endocrine disturbances in visceral fat tissue are the first stage of cardiovascular diseases following obesity. In other words, disturbance in hormones derived from adipose tissue such as adiponectin is the most important factor for disturbance in metabolism (4).
Adiponectin has anti-inflammatory effects and increases insulin sensitivity (4). Researchers are looking for the best and fastest way to adjust the calories consumed and taken in, so that by reducing the fat weight, the death rate caused by such diseases can be reduced (3). It seems that exercise training with the mechanism of increasing the activation of hormone-sensitive lipase, increasing the release of triglycerides and transferring them into mitochondria for metabolism can lead to a decrease in visceral fat weight (5). Regular exercise can inhibit pro-inflammatory factors by increasing the release of adiponectin from fat tissue and have beneficial effects on physical health (6). Studies show that various types of training (high intensity interval traini
 ng and low intensity continuous training) reduce calorie intake, reduce visceral fat weight and improve aerobic capacity in animal models fattened by high-fat food and suffering from diabetes (3). Also, long-term aerobic exercise training together with controlling calorie intake increased adiponectin to leptin ratio in overweight elderly (4). In Ouerghi et al.'s study, the results showed that although high intensity interval training improved insulin sensitivity and reduced inflammatory factors, no significant change was observed in adiponectin and omentin levels in overweight healthy young men (7).
ng and low intensity continuous training) reduce calorie intake, reduce visceral fat weight and improve aerobic capacity in animal models fattened by high-fat food and suffering from diabetes (3). Also, long-term aerobic exercise training together with controlling calorie intake increased adiponectin to leptin ratio in overweight elderly (4). In Ouerghi et al.'s study, the results showed that although high intensity interval training improved insulin sensitivity and reduced inflammatory factors, no significant change was observed in adiponectin and omentin levels in overweight healthy young men (7). On the other hand, considering the conflicting results regarding the effect of training caused by the duration, intensity and type of training, researchers have suggested the use of a proper diet and medicinal plants in addition to exercise training for greater efficiency (8, 9). Among the natural products, Curcumin (Cu), which is the most important component of turmeric, has been used in the treatment of many diseases for a long time, consequently this polyphenol has favorable biological effects such as anti-cancer, anti-diabetes, lowering blood sugar, improving liver enzymes, and improving some symptoms of metabolic syndrome (10). Also, researchers have suggested that the consumption of Cu can suppress pro-inflammatory and inflammatory cytokines in fat tissue and prevent the occurrence of some diseases from this pathway (11). Regarding the effect of Cu on fat tissue metabolism, researchers in a meta-analysis study on clinical trials showed that this natural antioxidant reduces Body Mass Index (BMI), decreases weight, and increases adiponectin in metabolic syndrome and related diseases (12). In addition, in a review study, some other researchers showed that the consumption of Cu leads to the inhibition of inflammatory factors in fat tissue and is also known as an anti-obesity factor (13).
Considering the conflicting results regarding the type and intensity-dependent effects of training on adiponectin changes, as well as the presence of an effective anti-obesity substance without side effects such as Cu, it seems that few studies have addressed the simultaneous mechanism of the use of Aerobic Training (AT) and Cu consumption in visceral fat tissue, and thus investigating the simultaneous effect of these two interventions can provide more comprehensive information for obese and overweight individuals to lose weight and reach fitness. Given the limited information in this regard, the aim of this study was to investigate the effect of six weeks of AT and Cu consumption on adiponectin gene expression levels in the visceral adipose tissue and some anthropometric characteristics of rats fed with a High-Fat Diet (HFD).
Materials and Methods
Preparation and maintenance of animals
In this experimental and fundamental study, 50 male rats with an age range of 8-10 weeks and a weight range of 180-220 grams were prepared from the breeding and reproduction center of Islamic Azad University of Zanjan branch. Throughout the research period, the ethical principles of working with animals were conducted based on the Helsinki Agreement and under the supervision of the ethics committee of Shahrood University of Medical Sciences with the approved code IR.SHMU.REC. 2018.87.
It is worth noting that all over the research period, the animals were kept under standard conditions in terms of light (12:12 hours of light and darkness), temperature (22-24°C), and relative humidity (55-60%). The animals were kept in washable polycarbonate cages and had ad libitum access to water and special food for rats. Also, sterile grated wood was used to absorb moisture and urine on the bottom of the cages. the rats were randomly divided into five groups, including: (1) healthy control (HC), (2) High-Fat Diet control (HFD), (3) consumption of curcumin + high-fat diet (HFD+Cu), (4) aerobic training + high-fat diet (HFD+AT), and (5) aerobic training + curcumin + high-fat diet (HFD + Cu + AT).
Preparation of high-fat diet (HFD)
In this research, high-fat diet emulsion was prepared so that each kilogram of it contained 400 grams of corn oil, 150 grams of sucrose, 80 grams of whole milk powder, 100 grams of cholesterol, 2.5 grams of multivitamins, 36.5 grams of Tween 80, 31 grams of propylene glycol and 10 grams of salt. Food and water were freely available for all rats, however the high-fat diet groups were given 1.5 mg/kg of body weight of this emulsion each day by gavage (14).
Aerobic training protocol
After grouping and adaptation, the rats were initially introduced to the laboratory animal treadmill. Habituation took place daily for 10 minutes at a speed of 8 m/min for one week. Also, to calculate the maximum running speed, which is equivalent to the maximum oxygen consumption in rats based on studies, the protocol of Natio et al. (2001) was used. Next, the relative intensity of endurance training in this research was considered equal to 75% of the maximum oxygen consumption; for this purpose, the incline of the treadmill at the beginning of the training period was considered 15%. Also, the AT program in this research started with a speed of 25 m/min for 10 minutes and reached 30 m/min for 50 minutes in the sixth week. In addition, rats in this research ran for 5 minutes at a speed of 10-15 m/min on a zero-degree slope at the beginning and end of the training to warm up and cool down (15). A better description of the training protocol is presented in Table 1 (15).

Curcumin consumption
In this study, rats received 1/5 g/kg daily by gavage. To this end, the Cu manufactured by Sigma-Aldrich (CAS Number: 458-37-7) was ordered to be prepared (16).
Dissection and sampling
After the end of the course and 48 hours after the last training session, rats fasted for 12 hours using a desiccator, and were anesthetized with a combination of 90 mg/kg ketamine and 10 mg/kg xylazine. After completing anesthesia and making sure of unconsciousness through tests of pain sensation and foot pressing and observing no pain reflex, the weight of the rats was initially measured using a digital scale with an accuracy of 0.01 grams, then the abdominal cavity of the rats was split with a scalpel and after discarding the excess tissues, all the visceral fat tissues, including the fat tissues around the intestines, the abdominal cavity and the fat tissues attached to the sides and back of the rats, were carefully extracted. The weight of all visceral adipose tissues was measured using a digital scale and then the tissues were placed in special tissue storage microtubes containing RNA Later. It is worth mentioning that the samples were immediately placed at -70°C and kept for the next steps.
Adiponectin gene expression measurement method
To measure adiponectin gene expression levels, the visceral fat tissue sample was first homogenized, then RNA extraction was performed using the recommended protocol in the special RNA extraction kit called plus-RNX manufactured by Sina Gene Company. To ensure the percentage of RNA purity, a spectrophotometer was used at wavelengths of 200 to 500 nm. After extracting and ensuring the purity of RNA, the samples were prepared for cDNA synthesis. For this purpose, the protocol recommended in the cDNA synthesis kit made by Takara Company in Japan was used. Next, the NCBI site was used to design leptin and adiponectin primers, and the primers were designed based on the guide available on this site. Also, to ensure the efficiency of the designed primers, the online software available on the NCBI website was used. After determining the efficiency of the primers, the reverse transcription reaction was performed using a Real Time-PCR device. Also, internal control gene Beta-2-Myoglobulin (B2M) was used to quantify the data, and after the completion, the formula 2-ΔΔCt was used to quantify the data according to the expression threshold cycle of the samples.
Data analysis procedure
In this research, the mean and standard deviation were first reported in the descriptive statistics, and then the Shapiro-Wilk test was used to check the normality of the data distribution. Next, to check the difference between groups in the BMI variables, one-way analysis of variance was used along with Tukey's post hoc test. Also, to analyze the pre-test and post-test data of the weight variable, and to remove the pre-test effect, covariance test and Bonferroni's post hoc test were used. In addition, the data of this research were analyzed using GraphPad PRISM 3.8.3 software at a significance level of P≤0.05.
Results
The results of one-way analysis of variance showed a significant difference in weight (P=0.001 and F=53.48), BMI (P=0.003 and F=4.61) and adiponectin gene expression (P=0.001 and F=5.60) in the research groups.
The results of the Bonferroni's post hoc test showed that the post-test weight levels in the HFD group were significantly higher than the HC group (P=0.001), however in the HFD+Cu (P=0.001), HFD+AT (P=0.001) and HFD+AT+Cu (P=0.001) groups, the levels were significantly lower than the HFD group. The levels were also significantly lower in the HFD+AT (P=0.001) and HFD+AT+Cu (P=0.001) groups than the HFD+Cu group. In addition, the levels were significantly lower in the HFD+AT+Cu group than the HFD+AT group (P=0.011) (Figure 1).
.PNG)
The results of Tukey's post hoc test showed that there was no significant difference in BMI levels in the HFD and HC groups (P=0.39). However, in the HFD+AT (P=0.02) and HFD+AT+Cu (P=0.008) groups, the levels were significantly lower than the HFD group. The levels were also significantly lower in the HFD+AT+Cu group than the HFD+Cu group (P=0.04) (Figure 2).
.PNG)
Adiponectin levels in the HFD group were significantly lower than the HC group (P=0.001). But in the HFD+Cu (P=0.001), HFD+AT (P=0.001) and HFD+Cu+AT (P=0.001) groups, the levels were significantly higher than the HFD group. The levels were also significantly higher in the HFD+AT (P=0.001) and HFD+Cu+AT (P=0.001) groups than the HFD+Cu group. In addition, the levels were significantly higher in the HFD+Cu+AT group than the HFD+AT group (P=0.001) (Figure 3).
.PNG)
Discussion
The results of the present study showed that the gene expression levels of adiponectin decreased following the high-fat diet, while the weight increased. On the other hand, AT increased the expression of adiponectin, while it decreased weight and BMI of rats exposed to high-fat diet. According to the studies conducted, the main cause of metabolic diseases following weight gain and obesity is the increase in insulin resistance. That is, an increase in insulin resistance exposes the cell to increased free radicals and damage with metabolic disorder. In addition, the increase in insulin resistance is associated with a decrease in the amount of adiponectin in adipose tissue, and simultaneously, the amount of leptin increases, which has an inverse relationship with adiponectin (17).
In addition, researchers have suggested that leptin increases following weight gain, and since it has a role in metabolic regulation, due to the destruction of its receptors on the cell surface, this hormone does not bind to its receptors properly, and as a result, a leptin resistance occurs making its function inadequate, and this activates T cells and stimulates the immune system. Subsequently, the stimulation of the immune system leads to an increase in the expression of inflammatory cytokines; on the other hand, adiponectin from AMP-Activated Protein Kinase (AMPK) leads to increased lipolysis and is directly associated with fat weight loss (18). Nonetheless, by increasing the metabolic demand to provide energy for cell life, exercise acts as a secretion regulator of leptin and adiponectin hormones; in other words, exercise activates hormone-sensitive lipase by phosphorylating protein kinases and leads to increased lipolysis in adipose tissue, thereby increasing the catabolism of fats in cells that need energy. As the diameter of fat vacuoles decreases in adipose cells, insulin sensitivity increases, and this is associated with a decrease in leptin expression and leptin resistance in other cells, and since there is an inverse relationship between adiponectin and leptin, an increase in nuclear transcription proteins leads to an increase in the gene expression and transcription of adiponectin (19). In line with the present study, in a review and meta-analysis, researchers stated that exercise increased adiponectin in 2996 people (20). Also, 20 weeks of caloric restriction training and exercise led to weight loss, reduction of body fat percentage and increase of plasma adiponectin in obese women (21). On the other hand, in a study, researchers showed that although 12 weeks of combined training improved BMI, reduced body fat percentage, increased net body mass and improved glucose metabolism, it did not have a significant effect on adiponectin levels in obese children (22). Banaii Broojeni et al showed that a progressive resistance-training program could prevent the loss of lean body mass and improve the reduction of total and abdominal fat mass and insulin resistance in overweight adult men, but it had no significant effect on adiponectin (23). It seems that the frequency and type of training were difference between our study and the above studies. Because the training performed in Jeon et al.'s study comprised two sessions per week, but in our study, they comprised five sessions per week (22). Also, in another study, the researchers stated that 6 months of AT, four sessions a week and each session 45 minutes with an intensity of 65 to 80% of the peak oxygen consumption increased insulin sensitivity, however there was no significant difference between adiponectin levels and body fat percentage in the training and control groups (24). These researchers concluded that exercise training depending on the amount of the effect on fat mass can change adiponectin levels. Therefore, it seems that changes in adiponectin levels following exercise are dependent on the changes in the fat percentage and its lipolysis.
The results of the present study showed that the consumption of Cu increased the expression of adiponectin and decreased the weight of rats exposed to high-fat diet. Based on the studies conducted, it seems that Cu by upregulating mechanism of AMP-Activated Protein Kinase (AMPK) and by activating Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) co-activator, increasing hepatic protein kinase B, activating deacylation-dependent proteins such as sirtuin 1 and 3 (SIRT1/3), leads to a decrease in inflammatory cytokines through affecting monocyte chemoattractant protein-1, increasing lipolysis, and increasing mitochondrial biogenesis, and results in an increase in adiponectin expression , decrease in fat weight and finally increase in lipolysis (12). In their study, Akbari et al stated that Cu consumption led to a decrease in BMI, waist to hip circumference ratio, body fat percentage, and leptin levels, and an increase in adiponectin in children with metabolic syndrome and related diseases (12). It was also stated in Bradford's study that Cu consumption had a beneficial effect on obesity and related diseases through lipolytic and anti-inflammatory biological activities (13). Also, consistent with the findings of this study, in a review study, the researchers stated that the consumption of Culed to an increase in adiponectin levels (25). Also, the results showed that simultaneous AT and Cu consumption increased the expression of adiponectin and decreased the weight and BMI of rats exposed to high-fat diet. In addition, the effect of training on the increase of adiponectin and decrease of weight and BMI was higher than Cu consumption.
According to the studies, it seems that both exercise training and Cu consumption through similar mechanisms of AMPK activation, PPAR-γ and SIRT1/3 phosphorylation (12, 18), reduction of inflammatory factors and improvement of immune system function (13,19) lead to increased expression of adiponectin. In addition, it seems that frequency-dependent exercise training and the basic levels of body fat percentage can affect adiponectin levels. On the other hand, the use of Cu in longer periods and higher doses has a more favorable effect on the metabolism of fat tissue. Regarding the simultaneous effect of training and Cu consumption, researchers have shown that IL-10 and Brain-Derived Neurotrophic Factor (BDNF) levels increased and IL-6 levels decreased significantly. However, the antioxidant effect of Cu was greater than training so that it caused a greater decrease in MDA and C-reactive protein in women with metabolic syndrome (26). In another study researchers showed that Swimming and curcumin consumption simultaneously can significantly moderate apoptosis caused by ethanol in the heart tissue (27). Also, in a study, combined training along with Cu consumption increased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (PGC1-α), Forkhead box protein O1 (FOXO1), and decreased cardiac tissue atrophying protein in obese Wistar rats (28). Likewise, in another study, researchers showed that combined training and Cu consumption improved fat metabolism in inactive middle-aged men (29). Therefore, it seems that exercise training and Cu consumption can be synergistically effective in increasing lipolysis, reducing body fat percentage, and increasing adiponectin.
Due to the effect of the frequency and intensity of exercise training and the affectability of the body's oxidation system to these factors, the lack of measurement in this study was one of the limitations of our study. Therefore, it is suggested to evaluate oxidant-antioxidant markers in future studies along with different exercise intensities. Also, the functional complexity of cytokines and adipokines derived from adipose tissue and the lack of measurement of some of them, such as leptin, are other limitations of the present study. Thus, it is suggested to evaluate leptin levels along with histological examination and the size of adipose tissue cells in the following studies.
Conclusion
It seems that curcumin consumption and exercise training both individually and synergistically can lead to weight loss and improve body mass index by improving adiponectin. Therefore, the use of these two (aerobic training and curcumin) is suggested in the conditions of metabolic disorders.
Acknowledgment
This research was conducted in the form of doctoral thesis of Ms. Zahra Hashemi Shiri at Islamic Azad University, Semnan branch. The authors hereby express their gratitude to the vice president of research of this university.
Funding source(s)
The researchers did not receive any financial support for this research.
Conflict of interest
The authors declare that there is no conflict of interest.
Type of Article: Original article |
Subject:
Health
Received: 2022/09/3 | Accepted: 2022/11/22 | Published: 2022/12/31
Received: 2022/09/3 | Accepted: 2022/11/22 | Published: 2022/12/31
References
1. Guerreiro VA, Carvalho D, Freitas P. Obesity, Adipose Tissue, and Inflammation Answered in Questions. J Obes. 2022; 252516. 1-11. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
2. Davari F, Alimanesh Z, Alimanesh Z, Salehi O, Hosseini SA. Effect of training and crocin supplementation on mitochondrial biogenesis and redox-sensitive transcription factors in liver tissue of type 2 diabetic rats. Arch Physiol Biochem. 2020;1-6. [DOI] [PMID] [Google Scholar]
3. Hosseini SA, Norouzi S, Rafiee N, Farzanegi P, Salehi O, Farkhaie F. Interactive Effects of Endurance Training and Crocin on Aerobic Capacity, Dietary Intake and Weight of High-Fat Diet-Induced Type 2 Diabetic Rats. J Nutr Sci Diet. 2018;4(3): 65-74. [view at publisher] [Google Scholar]
4. Senkus KE, Crowe-White KM, Bolland AC, Locher JL, Ard JD. Changes in adiponectin: leptin ratio among older adults with obesity following a 12-month exercise and diet intervention. Nutr Diabetes. 2022;12(1):1-7. [DOI] [PMID] [PMCID] [Google Scholar]
5. Ko K, Woo J, Bae JY, Roh HT, Lee YH, Shin KO. Exercise training improves intramuscular triglyceride lipolysis sensitivity in high-fat diet induced obese mice. Lipids Health Dis. 2018;17(1):1-7. [DOI] [PMID] [PMCID] [Google Scholar]
6. Babaei P, Hosseini R. Exercise training modulates adipokines dysregulations in metabolic syndrome. Sport Med Heal Sci. 2022; 20;4(1):18-28. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
7. Ouerghi N, Fradj MK Ben, Duclos M, Bouassida A, Feki M, Weiss K, et al. Effects of High-Intensity Interval Training on Selected Adipokines and Cardiometabolic Risk Markers in Normal-Weight and Overweight/Obese Young Males. A Pre-Post Test Trial. Biology (Basel). 2022;11(6):853. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
8. Hosseini SA, Hamzavi K, Safarzadeh H, Salehi O. Interactive effect of swimming training and fenugreek (Trigonella foenum graecum L.) extract on glycemic indices and lipid profile in diabetic rats. Arch Physiol Biochem. 2020;1-5. [DOI] [Google Scholar]
9. Nezafat Absardi M, Shadmehri S, Salehi OR, Hajisadeghi H. The Interactional Effects of Endurance Training and Aloe Vera Gel on Alanine Aminotransferase and Aspartate Aminotransferase levels in Diabetic Rats. Yafte. 2018; 20 (1) :99-111. [view at publisher] [Google Scholar]
10. Kasprzak-Drozd K, Oniszczuk T, Gancarz M, Kondracka A, Rusinek R, Oniszczuk A. Curcumin and Weight Loss: Does It Work? Int J Mol Sci. 2022;23(2):639. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
11. Varì R, Scazzocchio B, Silenzi A, Giovannini C, Masella R. Obesity-associated inflammation: does curcumin exert a beneficial role? Nutrients. 2021;13(3):1021. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
12. Akbari M, Lankarani KB, Tabrizi R, Ghayour-Mobarhan M, Peymani P, Ferns G, et al. The effects of curcumin on weight loss among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:649. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
14. Vajragupta O, Boonchoong P, Watanabe H, Tohda M, Kummasud N, Sumanont Y. Manganese complexes of curcumin and its derivatives: evaluation for the radical scavenging ability and neuroprotective activity. Free Radic Biol Med. 2003;35(12):1632-1644. [view at publisher] [DOI] [PMID] [Google Scholar]
15. Noura M, Arshadi S, Zafari A, Banaeifar A. The effect of running on positive and negative slopes on TNF-α and INF-γ gene expression in the muscle tissue of rats with Alzheimer's disease. J Basic Res Med Sci. 2020;7(1):35-42. [DOI] [Google Scholar]
16. Meshkibaf MH, Maleknia M, Noroozi S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund's adjuvant inflammation-induced male rats. J Inflamm Res. 2019;12:241. [DOI] [PMID] [PMCID] [Google Scholar]
17. Li X, Zhang D, Vatner DF, Goedeke L, Hirabara SM, Zhang Y, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci. 2020;117(51):32584-32593. [DOI] [PMID] [PMCID] [Google Scholar]
18. Einollahi N, Alirezaee A. A review of adipose tissue hormones and their functions in the body. Lab Diagnosis. 2016;8(33):23-34. [view at publisher] [Google Scholar]
19. Shahidi F, Pirhadi S. The effect of physical exercise and training on serum leptin levels. Razi J Med Sci. 2014;21(126):1-14. [Google Scholar]
20. Becic T, Studenik C, Hoffmann G. Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: systematic review and meta-analysis of randomized controlled trials. Med Sci. 2018;6(4):97. [DOI] [PMID] [PMCID] [Google Scholar]
21. Wang X, You T, Murphy K, Lyles MF, Nicklas BJ. Addition of exercise increases plasma adiponectin and release from adipose tissue. Med Sci Sports Exerc. 2015;47(11):2450. [DOI] [PMID] [PMCID] [Google Scholar]
22. Jeon J-Y, Han J, Kim H-J, Park MS, Seo DY, Kwak Y-S. The combined effects of physical exercise training and detraining on adiponectin in overweight and obese children. Integr Med Res. 2013;2(4):145-50. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
23. Banaii Broojeni J, Keshavarz S, Zakavi I. Effect of progressive resistance training on insulin resistance and plasma adiponectin concentration in overweight men. Jorjani Biomed J. 2019;7(1):51-64. [view at publisher] [Google Scholar]
24. Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Metab. 2002;283(4): 861-865. [view at publisher] [DOI] [PMID] [Google Scholar]
25. Clark CCT, Ghaedi E, Arab A, Pourmasoumi M, Hadi A. The effect of curcumin supplementation on circulating adiponectin: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr Clin Res Rev. 2019;13(5):2819-2825. [view at publisher] [DOI] [PMID] [Google Scholar]
26. Osali A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol Metab Syndr. 2020;12(1):1-7. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
27. Abdolhamid Tehrani M. Study the Effect of Swimming Training and Curcumin on Reduction of BAX and P53 proteins and Increase in BCL2 protein in Heart Tissue of Rats During Withdrawal of Excessive Ethanol Consumption. Jorjani Biomed J. 2022; 4: 1-11. [view at publisher] [Google Scholar]
28. Salkovic-Petrisic M, Perhoc AB, Homolak J, Knezovic A, Osmanovic Barilar J, Riederer P. Experimental Approach to Alzheimer's Disease with Emphasis on Insulin Resistance in the Brain. Handb Neurotox. 2021;1-52. [view at publisher] [DOI] [Google Scholar]
29. Sedaghat H, Fattahi Bafghi A, Emami A. The effect of combined exercise training course with curcumin supplementation on lipid profiles of inactive middle-aged men. Iran J Diabetes Obes. 2018;10(2):61-6. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





